O-Carboranes
Molecular formula: C2H12B10
Molecular weight: 144.22700
CAS NO: 16872-9-6
Melting point: 285-287 ℃
Water soluble: insoluble in water
Stability: stable at normal temperature and pressure
Character: light yellow or white crystal
Storage conditions: at room temperature, airtight and away from light, ventilated and dry.
Avoided material: oxide
Product description: Electron-deficient boron cage compounds with one or more carbon atoms as ligands in a borane skeleton.
Detection method: gas chromatography-mass spectrometry instrument
Product application: functional rubber precursors, 3D printing, aerospace fuels, intermediates

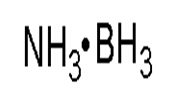
.png)